Some states are not getting the types and amounts of Covid-19 testing supplies that they’ve requested from the Trump administration, according to a report by Senate Democrats.
The report, released yesterday, sheds light on the problems with testing in the U.S. since the early days of the outbreak. Although U.S. health officials are now performing around 600,000 tests each day, many states are still grappling with supply shortages and test result delays—hamstringing the U.S.’s ability to fight the pandemic.
The Democratic report from the Senate Health, Education, Labor, and Pensions Committee showed that states received wrong orders, delayed shipments, and in some cases, unusable supplies. One state said that as of June 3 that it hadn’t gotten clear information on where to direct supply requests or what the federal government could fulfill.
One state still hasn’t received its full May allocation of swabs and tubes of viral transport media, the liquid to carry Covid-19 samples, according to the report. Another didn’t obtain its first usable delivery of swabs until late May, the report said. One state received swabs that were labeled cotton, but the manufacturer said they were polyester. Another got shorter nasal swabs, even though it was only using longer nasopharyngeal swabs.
The report said “one of the largest lab companies in the U.S. said the country could reach a million tests per day only if there was a real, clear, and concise plan for how to achieve that.” The panel report was based on interviews with Covid-19 test manufacturers, national health organizations, and public health officials from eight states and the District of Columbia.
But Brett Giroir, assistant secretary for health at the Department of Health and Human Services, pushed back on the report, saying the accusations “generally represent mismanagement and miscommunication at the state level.”
Giroir said in a statement that all supplies sent were authorized or approved by the Food and Drug Administration. “There were extensive pre-notification and notification processes in place to provide assurance of the usability and safety of the materials within those packages,” he said.
Giroir, who is overseeing the administration’s efforts to ramp up testing, said on June 3 the federal government planned to give states up to 20 million swabs and 20 million tubes of the fluid in May and June, and 100 million of each through at least December. But states and manufacturers said they’re expecting shortages in plastics to make the tubes, possibly limiting the ability to perform more tests. Read more from Shira Stein.
Congressional Virus Efforts
Unequal Effects Due to Pandemic: The House Homeland Security Emergency Preparedness, Response, and Recovery Subcommittee scheduled a hearing today on the unequal effects of the Covid-19 pandemic.
Vaccine Manufacturers to Testify: The House Energy and Commerce Oversight and Investigations Subcommittee will hold a hearing July 21 with Covid-19 vaccine manufacturers, according to a statement. Officials from AstraZeneca, Johnson & Johnson, Merck, Moderna and Pfizer will testify at the hearing.
Appropriators Set Top-Line Levels: The House Appropriations Committee has set top-line spending levels for all 12 of its fiscal 2021 government funding bills. The panel advanced the spending allocations on a 29-21 vote. The set provides for $1.3 trillion in discretionary spending, although House Democrats have also included $247.4 billion in extra emergency money in response to the pandemic and for veterans’ health care. House appropriators aim to finish all 12 markups before next Wednesday, Chairwoman Nita Lowey (D-N.Y.) said, Jack Fitzpatrick reports.
The committee will mark up the Labor-HHS-Education bill on Monday.
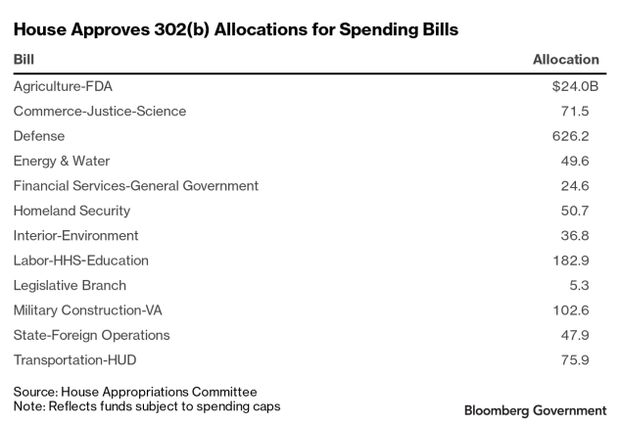
- The panel yesterday also advanced the fiscal 2021 Agriculture-FDA spending bill. It adopted Subcommittee Chairman Sanford Bishop’s (D-Ga.) amendment that grants the FDA the legal authority to order the recall of both over-the-counter and prescription medicines and products that might cause serious harm and death. Bishop said the FDA unofficially supports the amendment, Megan Boyanton reports.
- The panel yesterday also advanced the spending bill for the State Department and foreign operations programs that would require the administration to send $200 million to the World Health Organization. The bill would provide $66 billion in discretionary funding, an $8.5 billion increase over fiscal 2020. The bill also would provide more than $10 billion for a variety of pandemic-related emergency programs, Fitzpatrick reports.
More on the Pandemic
Trump to Sign Orders on Prescription Drugs: White House Chief of Staff Mark Meadows, in an interview with Lou Dobbs on Fox Business Network, said President Donald Trump is planning to sign three executive orders on drug pricing. Meadows said he was talking with Trump about the orders yesterday, without providing more details. His remark came as Sen. Chuck Grassley (R-Iowa) is planning to push for a vote on a drug pricing measure (S. 2543), following Gilead Sciences’ announcement that it’s pricing a treatment of remdesivir at $2,340. Read more on that effort by Alex Ruoff.
Decontaminating Masks Raises Safety Concerns: The use of decontaminated masks has raised safety concerns among nurses, but mask manufacturers such as 3M, and government agencies, such as the Occupational Safety and Health Administration, say the sterilized masks are safe, although they’d prefer new ones to be worn. “We’ve seen a rush to embrace these decontamination processes,” said Jane Thomason, lead industrial hygienist for National Nurses United. “They’ve not been studied. They’ve not been fully evaluated.” Read more from Bruce Rolfsen.
States Get Tough on Virus Rule Breakers: Governors and city leaders who want people to stay six feet apart, wear masks, and change their business practices to slow the spread of the coronavirus are turning to fines, mandates and suspending licenses. Faced with surging case numbers, defiant businesses, and quarantine-fatigued residents, some officials have increased enforcement efforts throughout the country. Read more from Brenna Goth.
Private Equity’s Back-Door Path to Federal Relief: The private equity world’s massive push into U.S. health care is offering deep-pocketed investors a boost from taxpayer funds meant to help small businesses. Buyout firms were largely excluded from tapping the federal bailout money as the pandemic shutdown businesses. But a trove of data from the Paycheck Protection Program made public earlier this week lists millions in loans to medical and dental practices that work with ventures controlled by private equity—setting up those investments to benefit too. Read more from Heather Perlberg.
Moderna in Pact to Help Supply Vaccines: Moderna joined with Laboratorios Farmaceuticos Rovi to help supply its Covid-19 shot, one of the leading vaccine candidates for the disease, as drug companies race to produce any successful inoculation as soon as possible. The Spain-based pharmaceutical company will provide vial filling and packaging capacity to Moderna, according to a statement yesterday, as Moderna prepares to produce hundreds of millions of doses for markets outside the U.S. starting in 2021. Thomas Gualtieri has more.
Miami-Dade Hospital, ICU Patients Hit Record: Miami-Dade County, Florida’s most populous, again reported the highest numbers of coronavirus patients in intensive-care and hospitals generally since at least early April. Miami-Dade has 1,688 people with Covid-19 in hospitals, an increase of 32 from the day before. The number of virus patients in intensive-care unit beds rose to 358 from 343. Covid-19 patients on ventilators jumped to 184 from 175 a day earlier and was at the highest since April 20, according to the county’s daily report. Read more from Jonathan Levin.
More Headlines:
What Else to Know Today
SCOTUS Ruling Said May Worsen Disparities: The Supreme Court’s decision to uphold the Trump administration’s regulations that widen an employer’s ability to deny workers birth control coverage could have dire consequences for Black and low-income people, health advocates warned. They and health law scholars argue the ruling could exacerbate racial inequalities that already exist in health care by expanding barriers to critical preventative care and family planning that can improve the overall health of marginalized people. Lydia Wheeler has more.
More Headlines:
To contact the reporter on this story: Brandon Lee in Washington at blee@bgov.com
To contact the editors responsible for this story: Giuseppe Macri at gmacri@bgov.com; Zachary Sherwood at zsherwood@bgov.com; Michaela Ross at mross@bgov.com
"bad" - Google News
July 10, 2020 at 05:15PM
https://ift.tt/3iOdRHo
HEALTH CARE BRIEFING: Feds Gave States Bad Supplies, Report Says - Bloomberg Government
"bad" - Google News
https://ift.tt/2SpwJRn
https://ift.tt/2z7gkKJ

No comments:
Post a Comment